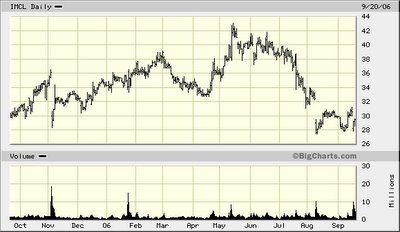
On Wednesday morning, prior to being elected to the Board of Directors at Imclone Systems, Inc. (IMCL-$29.50), billionaire investor Carl Icahn said in an open letter delivered to Chairman David Kies that he expected Kies to make good on a promise to give up his job at the biotechnology company:
Dear David:
When you offered me a directorship at ImClone in August, we discussed and I thought you indicated that you would not continue to be the Chairman of the Board of the Company. I also told you that it would be a mistake to give the Interim CEO a long-term contract, given that he has little or no expertise in biotech companies. I asked that you at least allow the new Board to make this decision. Nevertheless, without warning, ImClone entered into a multi-million dollar contract with the Interim CEO. You have also indicated that you will not be willing to give up your chairmanship at today's meeting.
On January 24, 2006, Joseph L. Fischer, 56, was named Interim Chief Executive Officer of the Company. Icahn is correct in his first assertion that this interim CEO “has little or no expertise in biotech companies.” In his thirty-year career, Mr. Fischer has served in a variety of senior management positions, most notably with the personal care division of Johnson & Johnson and Dial Corp’s global Consumer Products group. Mr. Fischer became a CPA in New York. In 1972, he graduated with a Bachelor of Science in Accounting at Penn State University.
The Company entered into an employment agreement with Mr. Fischer on August 23, 2006. Effective immediately, Fischer will receive $45,000 per month in base salary while he serves as Interim CEO. If Mr. Fischer is still employed by Imclone on the date that bonuses for fiscal 2006 are paid, he will receive a bonus of no less than $500,000 and, if the Company employs him on December 31, 2007, he will receive a $500,000 retention bonus.
If the Company without cause terminates Fischer’s employment, or in the event he terminates his employment with good reason following a change in control of the Company, Mr. Fischer will be entitled to a lump sum cash severance payment of not less than $500,000. [Ed. note. This is on the cheap as compared to many CEO resignations that we have commented on in the past.]
The Compensation Committee also (unanimously) granted Mr. Fischer stock to purchase 100,000 shares of the Company's Common Stock at an exercise price equal to the closing price of the on the date of grant (i.e., February 28, 2006). Contrary to Icahn’s noted umbrage, the exercise price for Fischer is $38.30 per share—almost nine dollars higher than the current share price of the Common Stock.
Now that I am becoming a director of ImClone, I am asking you again for the good of ImClone and its stockholders to give up your position as Chairman of the Board. Given what I consider the sorry record of the Company under your watch, it is time for you to step aside and allow someone else to be elected. You have even admitted to me that the board has done a bad job. ImClone has been without effective leadership for almost three years.
During your tenure, ImClone has suffered as a result of its inability to attain the leadership position it should enjoy as an important biotechnology company. Most importantly, a great disservice has been done not only to stockholders, but, potentially, to cancer patients as well, by ImClone's apparent passivity in pushing to start appropriate trials in first-line colon cancer and other indications.
Erbitux is Imclone’s only product on the market (in two tumor types—colorectal cancer and head & neck tumors). For the six-months ended June 30, 2006, the drug generated total sales of approximately $395.0 million, as compared to $178.1 million in the prior year period.
During your tenure, I believe that commercialization has suffered, trials have not been sufficiently pursued…. the Company has not provided its stockholders the performance that they deserve. Rumors abound about employee dissatisfaction and probable defections.
A Phase III clinical trial is underway for the first-line treatment of advanced stage pancreatic cancer. Trial results will probably be available in the 1H:07. Additional Phase 3 trials are underway for first-line and second-line use in advanced non-small cell lung cancer (NSCLC).
Unfortunately, disappointing results for the first-line use of Erbitux with chemotherapy in colorectal cancer patients were released in June 2006, for survival results did not differ significantly between the two patient groups.
When Icahn talks “Sorry Record and performance that stockholders deserve,” in our view he is referencing Icahn Group’s dismal total return on its investment in Imclone. Look at the 52-week drop in the price of the Common Stock, and you will see one angry man sliding downhill with the Company’s share-net price.
As of the time of the Board meeting, the Icahn Group beneficially owned approximately 11.7 million shares, or 13.8% of the outstanding shares, at an aggregate cost of $390.5 million (including commissions)—or $33.38 per share.
During your tenure, ImClone hired a President and CEO who was totally the wrong person for the position and it took you many many months to recognize this and replace him. His replacement lasted only a few months. Now, ImClone has another interim CEO and his permanent replacement is nowhere on the scene. To make matters worse, you even rewarded him with a favorable contract increasing his compensation and making it more expensive to replace him. This has all occurred during the most critical period in the history of the Company in which its ability to exploit its lead in cancer treatment was being tested. Your regime has failed the test.
Daniel S. Lynch, 48, was the aforementioned “wrong person for the position.” He joined the Company in April 2001 as its Vice President, Finance and CFO. In February 2004, he was promoted from interim CEO to CEO, and continued to serve in this capacity (and as a Director) until his employment was terminated with the Company (effective as of November 10, 2005). Imclone paid Mr. Lynch a salary (and bonus) of $486,000 and $903,477 in FY 2005 and 2004, respectively. As part of his severance agreement, the Company made cash payment of approximately $2.8 million to Mr. Lynch.
Upon the departure of Mr. Lynch, Philip Frost, M.D., Ph.D., then Executive Vice President, Chief Scientific Officer of the Company, assumed the additional position of Interim CEO. Dr. Frost served in those dual positions until January 23, 2006, when Joseph L. Fischer was appointed to the position of Interim CEO. Dr. Frost remains with the Company in his previous position as Executive Vice President and Chief Scientific Officer. In addition to the compensation for his service as Executive Vice President and Chief Scientific Officer, Dr. Frost received $16,818 in base salary in his capacity as Interim CEO (in addition to his salary/bonus of $510,077).
During your tenure, ImClone's meaningful lead relative to potential competitors has shrunk considerably and ImClone has suffered reversals such as the loss of the patent suit in the past week. I cannot believe that there were not a number of opportunities to achieve a favorable settlement of the patent suit under your leadership. Now the suit has been lost.
While Chairman Vies fiddled, Imclone’s first-to-market advantage with its epidermal growth factor receptor (EGFR) inhibitor, Erbitux (Cetuximab), burned to the ground. Biotech giant Amgen (AMGN-$70.98) is awaiting word from the FDA to bring to market its own candidate for EGFR-expressing metastatic colorectal cancer, called Vectibix (Clinical Drug: Panitumumab).
After three years, on September 18, 2006, the U.S. District Court in NYC ruled that scientists associated with Yeda Research and Development were the sole owners of a patent (involving the method of using a monoclonal antibody in combination with chemotherapy) that ImClone Systems licenses from Sanofi-Aventis SA (SNY-$43.91).
Aside from appealing the court’s decision, one tactic Imclone could employ would be to negotiate an exclusive license for Erbitux with antineoplastic agents covered by the patent.
.
Outmaneuvered! Securing potential uses for its own EGFR-inhibitor, Amgen has already announced that it has secured licensing rights from Yeda Research.
The Company is facing additional intellectual property litigation, too, dealing with the production (manufacturing) of Erbitux in commercial quantities. On July 28, 2006, the Company’s motion for summary judgment seeking to dismiss all claims on the basis that the patent rights at issue were exhausted as a matter of law was denied by the courts. The plaintiffs, MIT and Repligen Corp. (RGEN-$3.08), will now take their case to trial.
[Ed. note. As management does not believe the patent litigation will have a material impact on its operations, no reserve has been established in the financial statements for any of the Yeda or MIT and Repligen actions]
You should recognize that your leadership of ImClone should come to an immediate end. The time has come for you to peacefully pass the baton to a successor who will be able to bring strong leadership back to ImClone. If you fail to do so, you will have thrown down the gauntlet and we will have to react accordingly.
Very truly yours,
Carl C. Icahn.
On January 24, 2006, the Company announced that the Board of Directors had engaged the investment bank Lazard to conduct, in conjunction with management, a full review of the Company’s strategic alternatives to maximize shareholder value—including a sale of the Company.
David M. Kies, 62, a Director of the Company since June 1996, was named Chairman in February 2004. Mr. Kies is a Partner of the New York-based law firm Sullivan & Cromwel, specializing in mergers and acquisitions.
Ironically, Mr. Kies’ and Icahn are on the same side of the street, for Kies’ stake in Imclone is underwater, too. In 2005, the Chairman of the Board was granted options to purchase 45,000 shares of Common Stock at an exercise price of $46.12 per share; with respect to 2006 service, he has been granted option to purchase 30,000 shares of Common Stock at $34.14 per share.
The time, however, may have come for Kies to “peacefully pass the baton.” After eight months of review, the Company announced its decision to remain independent, citing that the alternatives available, including bids received for the acquisition of the Company were too low, and did not match the Company’s intrinsic value (potential).
What is the value potential in owning Imclone Common Stock? The Company has a broad spectrum of innovative product candidates with potential application in multiple tumor types: (i) growth factor blockers for solid tumors and (ii) tumor angiogenesis blocker called vascular endothelial growth factor receptors (VEGFR). However, these biologics are still in preclinical or Phase 1 stages of development.
The Company is facing additional intellectual property litigation, too, dealing with the production (manufacturing) of Erbitux in commercial quantities. On July 28, 2006, the Company’s motion for summary judgment seeking to dismiss all claims on the basis that the patent rights at issue were exhausted as a matter of law was denied by the courts. The plaintiffs, MIT and Repligen Corp. (RGEN-$3.08), will now take their case to trial.
[Ed. note. As management does not believe the patent litigation will have a material impact on its operations, no reserve has been established in the financial statements for any of the Yeda or MIT and Repligen actions]
You should recognize that your leadership of ImClone should come to an immediate end. The time has come for you to peacefully pass the baton to a successor who will be able to bring strong leadership back to ImClone. If you fail to do so, you will have thrown down the gauntlet and we will have to react accordingly.
Very truly yours,
Carl C. Icahn.
On January 24, 2006, the Company announced that the Board of Directors had engaged the investment bank Lazard to conduct, in conjunction with management, a full review of the Company’s strategic alternatives to maximize shareholder value—including a sale of the Company.
David M. Kies, 62, a Director of the Company since June 1996, was named Chairman in February 2004. Mr. Kies is a Partner of the New York-based law firm Sullivan & Cromwel, specializing in mergers and acquisitions.
Ironically, Mr. Kies’ and Icahn are on the same side of the street, for Kies’ stake in Imclone is underwater, too. In 2005, the Chairman of the Board was granted options to purchase 45,000 shares of Common Stock at an exercise price of $46.12 per share; with respect to 2006 service, he has been granted option to purchase 30,000 shares of Common Stock at $34.14 per share.
The time, however, may have come for Kies to “peacefully pass the baton.” After eight months of review, the Company announced its decision to remain independent, citing that the alternatives available, including bids received for the acquisition of the Company were too low, and did not match the Company’s intrinsic value (potential).
What is the value potential in owning Imclone Common Stock? The Company has a broad spectrum of innovative product candidates with potential application in multiple tumor types: (i) growth factor blockers for solid tumors and (ii) tumor angiogenesis blocker called vascular endothelial growth factor receptors (VEGFR). However, these biologics are still in preclinical or Phase 1 stages of development.
.
At present, Imclone is a one-trick pony—named Erbitux. The Company is like McDonald’s when all it had on its menu was burgers, fries, and shakes. The difference is that McDonalds had a visionary leader, Ray Kroc.
Imclone has a CEO with no background in biotech. After yesterday—Imclone does have Carl Icahn, an activist stakeholder known for aggressively pushing for corporate changes to enhance stock value. Launching proxy fights is like getting dressed in the morning for financier Icahn. Recently, investors who followed Icahn into the maker of the hit Grand Theft Auto video-game series Take-Two Interactive (TTWO-$15.01) and Symantec (SYMC-$20.95), the No. 1 anti-virus software maker, were rewarded by gains of 27% and 18% in the price of the stocks, respectively, in just a few months.
Imclone has approximately $964 million, or $11.44 per share, in cash. Net the $844.2 million in total contractual cash obligations (mostly LT Debt, Interest Expense, and Operating Leases), or $(10.01), leaves a less-than-impressive $1.43 per share in cash. (However, the LT Debt does not come due—aside from designated events—until May 2009, 2014, or 2019.)
Despite the patent setback, the Company will probably reach a settlement and end up paying royalties (estimated at two –to – three percent of sales) to Yeda Research.
In our view, the bigger worry is Amgen’s Vectibix and Genentech’s (DNA-$79.17) VEGF-blocker, Avastin, which received FDA approval (in combination with chemotherapy) for Second-Line Metastatic Colorectal Cancer Patients in late June 2006.
Icahn is correct, current management is not up to the task of taking on this Sisyphean task.
If, however, Icahn can pull off a coup de`tat, and milk the cash cow (Erbitux), investors could be handsomely rewarded in the end. BUY and wait for the ‘Icahn Effect.’
At present, Imclone is a one-trick pony—named Erbitux. The Company is like McDonald’s when all it had on its menu was burgers, fries, and shakes. The difference is that McDonalds had a visionary leader, Ray Kroc.
Imclone has a CEO with no background in biotech. After yesterday—Imclone does have Carl Icahn, an activist stakeholder known for aggressively pushing for corporate changes to enhance stock value. Launching proxy fights is like getting dressed in the morning for financier Icahn. Recently, investors who followed Icahn into the maker of the hit Grand Theft Auto video-game series Take-Two Interactive (TTWO-$15.01) and Symantec (SYMC-$20.95), the No. 1 anti-virus software maker, were rewarded by gains of 27% and 18% in the price of the stocks, respectively, in just a few months.
Imclone has approximately $964 million, or $11.44 per share, in cash. Net the $844.2 million in total contractual cash obligations (mostly LT Debt, Interest Expense, and Operating Leases), or $(10.01), leaves a less-than-impressive $1.43 per share in cash. (However, the LT Debt does not come due—aside from designated events—until May 2009, 2014, or 2019.)
Despite the patent setback, the Company will probably reach a settlement and end up paying royalties (estimated at two –to – three percent of sales) to Yeda Research.
In our view, the bigger worry is Amgen’s Vectibix and Genentech’s (DNA-$79.17) VEGF-blocker, Avastin, which received FDA approval (in combination with chemotherapy) for Second-Line Metastatic Colorectal Cancer Patients in late June 2006.
Icahn is correct, current management is not up to the task of taking on this Sisyphean task.
If, however, Icahn can pull off a coup de`tat, and milk the cash cow (Erbitux), investors could be handsomely rewarded in the end. BUY and wait for the ‘Icahn Effect.’
Editor David J Phillips does not own any of the stock mentioned in this article. You can see his portfolio holdings in the sidebar. The 10Q Detective has a full disclosure policy.
No comments:
Post a Comment